BACKGROUND
Legumes are used as a break crop for cereals. Legume roots are capable of developing a symbiotic relationship with the root-nodule to form soil bacteria rhizobia. Rhizobia in legume root-nodules, fix atmospheric nitrogen (N2) and convert it into ammonia (NH3), making it available to the plant for growth. The amount of nitrogen (N) fixed by the legume has a strong association with the productivity of the crop. Legumes are capable of fixing between 100 to 200 kg N/ha in one growing season when rhizobia are plentiful in the soil, but it will fix no N if the correct species of rhizobia is absent.
The pulse legumes grown in Australia are exotic to our country; our soils do not have the necessary rhizobia to allow legumes to fix any N. Inoculation with cultures of rhizobia is essential the first time a pulse is grown. However, if legumes are not regularly grown, the population of rhizobia is known to decline in the soil over time. It may be necessary to re-inoculate with rhizobia the next time a pulse is grown if it has been more than six years since the last legume crop, so as to ensure that the legume will be able to fix N.
The N-rich vegetative residues and nodulated roots remaining in the soil after harvest of a legume crop increase the amount of organic N in the soil, making more N available to subsequent crops. As a result, growing legumes in low N soils can be beneficial. Given the increases in costs of N fertiliser, inoculating legumes can potentially provide an inexpensive way to increase the return of N to soils.
Legumes play an important role in improving subsequent cereal yields by breaking the cycle of cereal root diseases such as cereal cyst nematode (CCN) and take-all, and have been shown to improve soil structure in some environments. Furthermore, legumes can provide a good weed break as they allow the use of different herbicide groups to manage weed issues. As legumes are more commonly grown in the Wimmera, this project aims to determine whether inoculation was beneficial to crop growth and if so, where these benefits occurred.
TAKE HOME MESSAGES
-
good nodulation in the uninoculated treatments, and the absence of growth or yield responses to inoculation in this trial, indicated that pulses have been grown sufficiently often in this particular paddock (lentil in 2007 and pulses every two/three years previously) to maintain appropriately high rhizobial populations in the soil
-
as the numbers of rhizobia decline in the soil over time, consider inoculation based on,paddock history and length of time since growing the last legume crops. Pulses often benefit from inoculation if there has not been a legume grown in the paddock for six years
where rhizobial populations are low in the soil, inoculation of legumes at sowing is extremelyimportant to ensure adequate nitrogen fixation
AIM
To assess the impact of rhizobial inoculation on the performance of four different legumes: chickpeas, lentils, faba beans and peas in the Victorian Wimmera.
METHOD
Location: Rupanyup
Replicates: 2
Sowing date: 20 May 2011
Crop type/s: chickpeas, lentils, beans, peas
Fertiliser: 20 May 50kg/ha MAP (10% N, 21.9% P)
Herbicide:
14 July – Select® (500ml/ha) + Liase® (2%) + Hasten® (1%)
26 Aug – Brodal Options (150ml/ha) – lentils + inoculant and peas + inoculant plots only
Fungicide: 20 Sept – Prosaro® (300ml/ha) + Spreadwet® (0.25%)
Seeding equipment: BCG Parallelogram (knife point, press wheels, 30cm row spacings)
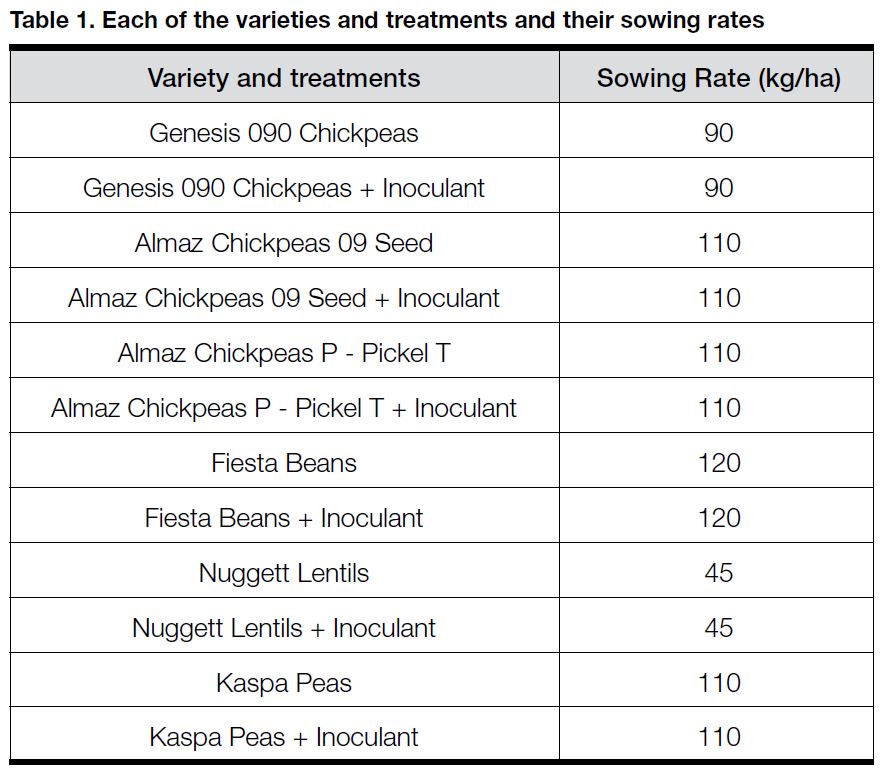
Four legumes (chickpeas c.v. Genesis and Almaz, lentils c.v. Nuggett, faba beans c.v. Fiesta and field peas c.v. Kaspa) were sown on 20 May on a Wimmera grey clay soil at Rupanyup. The trial was sown into a paddock following three years of cereal (barley, 2010; wheat, 2009; wheat hay, 2008.) The last legume (lentils) was sown in 2007. Due to the high stubble load from the 2010 barley crop, the decision was made to burn. The starting available soil N in the paddock was 109kg/ha, sampled to a depth of 1m.
Plots (1.8 x 12m) were pegged out and treatments replicated twice. Treatments were inoculated using appropriate Novozymes commercial peat inoculants and the trial sown immediately using the sowing rates shown in Table 1. Inoculated and uninoculated plots were sown side-by-side to facilitate visual observations.
The site had a very heavy weed burden, with volunteer barley grass, prickly lettuce, marshmallow and wild radish occurring through all plots. Mouse infestation was moderately high, with active holes observed early in the growing season. Baiting occurred on a number of occasions to reduce the risk of damage to the crop.
Assessments were carried out on all crops, including plant counts after emergence and evaluation of root nodulation at flowering. Shoot dry matter production was sampled during mid-pod fill around the time of peak biomass. These samples were used to determine the amount of N fixed with a technique based on the analysis of heavy-stable isotope of N, 15N. Unfortunately, the results of these analyses were not available at the time of writing. The trial was harvested on 6 December and samples were processed in the lab.
RESULTS
Crop Emergence
There were no significant differences in emergence between the inoculated and uninoculated treatments. There were substantial emergence issues due to a heavy weed burden in the paddock, as well as moderately high mouse activity. The Genesis 090 chickpeas and Nuggett lentils were most affected by mice.
The use of P-Pickel T seed treatment, may reduce the viability of the inoculum, but in this trial, the seed treatment did not affect emergence.
Above-ground dry matter production
There were no significant differences in shoot dry matter production between the inoculated and uninoculated treatments.
Nitrogen fixation
Examination of excavated roots on 6 September 2011 indicated reasonable nodulation (greater than 5-10 nodules per root system) in both the inoculated and uninoculated treatments for lentils, field peas, faba beans and chickpea. These initial results suggested that indigenous populations of rhizobia in the soil at sowing were probably sufficiently high to ensure successful nodulation and N fixation. We hope to be able to confirm this when the 15N analyses of all treatments is completed.
Grain Yield
There was no affect of inoculation on yield for any of the crop types tested. Use of P-Pickel T seed treatment did not affect yield of chickpeas regardless of inoculation treatment.
INTERPRETATION
The trial site at Rupanyup had a history of frequent legumes over the past decade, with pulses forming part of the rotation every two to three years. The last legume crop (lentils) was grown in 2007. The lack of obvious differences in the degree of root nodulation and the absence of significant growth or yield responses to the inoculation treatments in this trial indicate that pulses have been grown sufficiently often to maintain rhizobial populations in the soil.
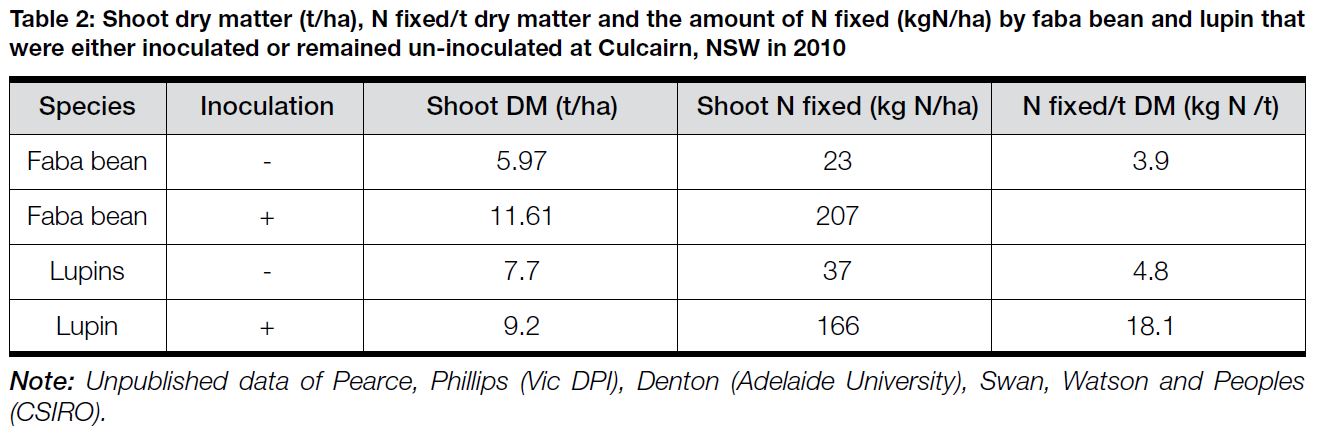
Where rhizobial populations are low in the soil, inoculation of the legumes at sowing is extremely important to ensure N fixation. In 2010, a similar trial was established at Culcairn in southern NSW to examine the effect of inoculation on both faba beans and lupins in a paddock that had not grown pulses for at least ten years. The results outlined in Table 2 indicate that there were significant increases in shoot dry matter (DM); the amount of N fixed over the growing season and the amount of shoot N fixed per tonne of dry matter accumulated for both the faba beans and the lupins when they had been inoculated.
In the Rupanyup trial, it is assumed that rhizobial numbers in the soil were adequate for the crop to reach its potential.
COMMERCIAL PRACTICE
Take into account the paddock history and length of time since the last legume crop when considering whether to inoculate their legume crops. Rhizobial numbers slowly decline in the soil over time if legumes are not regularly grown, and there is often a benefit of inoculation after a five to six year period between legume crops.
Keep in mind that while field peas, lentils, faba beans and vetch can nodulate with the same rhizobia species, a different rhizobia species is required for lupins and a different species again for chickpeas. It is important to look at the time interval between legumes that nodulate with the same rhizobial species.
ACKNOWLEDGEMENTS
This project was funded by BCG members and in association with CSIRO as part of GRDC project CSP000146.